Our Facilities
Keeping in view the WHO guidelines ZAFA adheres to the policy of dedicated and self contained facilities.

ZAFA Beta-Lactam Division is our dedicated facility for the manufacturing of Beta-Lactam (Penicillin only) products. The facility is built as per latest cGMP guidelines and is designed for facilitating better environmental control, material movement and personnel flow in order to avoid cross contaminations. The total covered area of this facility is around 40,000 square feet. This is a state of the art dedicated facility which is equipped to manufacture:
| • Dry Powder Injectables | • Tablets/Suspensions (requiring low Rh) | • Liquid Injectables |
| • Capsules | • Tablets | • Suspensions |
This facility is also equipped to manufacture products requiring a very low Relative humidity therefore specialized products can be manufactured utilizing this facility. ZAFA’s beta-Lactam is one of the few dedicated facility for the manufacturing of Beta-Lactam (Penicillins only) around the world. The capacity of this plant is for 1.5 million tablets, 1000Kg of Wet granulation, 1000 Kg of Dry Granulation, 300Kg of Film/Sugar coating, 1 million blisters, 400,000 capsules, 1000Kg of Dry Syrup, and 100,000 vials per day, single shift basis.
The facility utilizes latest Air Handling equipments to ensure the quality of air in the manufacturing and packaging areas. The vials are filled on latest monoblock Auto lines to ensure further adherence to Quality Control and GMP guidelines.
Since it is a dedicated facility there is almost zero chance of Cross Contamination which is a reason for major concern for all regulators and customers around the world.

ZAFA Cephalosporin Division is our dedicated facility for the manufacturing of Cephalosporin products. This facility is built as per latest cGMP guidelines and is designed for facilitating better environmental control, material movement and personnel flow in order to avoid cross contamination. This is a state of the art dedicated facility which is equipped to manufacture:
| • Dry Powder Injectables | • Capsules |
| • Tablets | • Suspensions |
ZAFA’s Cephalosporin facility is one of the few dedicated facility for the manufacturing of Cephalosporin products around the world.
The facility utilizes latest Air Handling equipments to ensure the quality of air in the manufacturing and packaging areas. This facility has a dedicated Quality Control and Quality Assurance facility which has been equipped with an in-house microbiology facility for carrying out microbiological tests of both the product and the manufacturing facility. The staff at this facility are also dedicated thus preventing even the slightest chance of cross contamination through personnel movement. Latest capsulation machines are used for encapsulation of our products.
Our Cephalosporin Division is another step towards quality, economy, commitment and compliance of our mission statement, which is to ensure the availability of quality drugs at affordable prices throughout the country and the world.

ZAFA Hormones Division is a dedicated facility for the manufacturing of Hormonal products. It has been built on latest WHO and cGMP guidelines and is one of the few dedicated facilities operating around the world. This facility is built over an area of 17,000 Square feet with additional Large Storage area for storage of materials. In addition to this it has also been inspected and approved by Mr. Bryan Hartley, Ex-Head of Inspection and Enforcement, MCA of the United Kingdom. Mr. David Peary, Deputy High Commissioner of the United Kingdom and Major General (retired) Mohammad Aslam, the Director General Health, Government of Pakistan jointly inaugurated this facility. This facility has dedicated equipments for production, packaging and Quality Control. Our hiring criteria at this unit are as per latest guidelines.
We are a socially responsible organization, thus not only the material waste is controlled through our Treatment plant but we also pay utmost importance to the air that is leaving our facility. HEPA filters is one of the systems that are used to ensure that the air leaving our facility is free from any hormonal content. The capacity at this facility is of 33 million cycles of combined Oral Contraceptives that can be increased to 60 million cycles (single-shift basis) within a short span of 6 weeks. In addition the capacity for manufacturing Emergency Contraceptives is around 1 million tablets per day. Our capacity for the manufacture of DMPA injections is around 2.5 million packs per annum (single-shift basis).
ZAFA Hormones exports contraceptive products to many countries around the world including Asia, Africa, South and Central America.
At ZAFA Hormones we are working on the latest cGMP guidelines and ensure consistency in our production results. Latest equipments are used to ensure content uniformity in tablets and thus we ensure that every tablet has the quantity of the raw material as stated. In addition results between different batches are compared to ensure the minimum possible variation of results between batches.

ZAFA Soft-Gelatin capsule facility is a dedicated world class facility for the manufacture of Pharmaceutical products requiring soft-gelatin formulations. Being dedicated it ensures no-cross contamination between products. As gelatin is an ideal medium for Growth therefore there is a requirement of keeping this facility dedicated. This facility is built over an area of 17,000 Square feet and has additional large storage area for storage of materials. This facility can be used to manufacture cardiovascular, vitamin and other preparations in Soft-Gelatin Capsules. It is an automatic facility and is built as per latest cGMP guidelines.
The plant consists of the gelatin melting tank, encapsulating machine, Stainless Steel Capsule conveyor, Tumbler driers and other auxiliary equipments. The soft-gelatin capsules are continuously formed and simultaneously filled by the encapsulating machine and carried by stainless steel conveyor into a tumbler drier for drying. This process ensures production of soft-gelatin capsules with different shapes and provides unique dosage presentation/shapes as per our marketing/customer requirements. The whole process is almost hands-free thus ensuring compliance to latest cGMP standards. The capacity of this plant is around 400,000 capsules per day that can be easily doubled in a short span of 3 months.
This facility is being used for meeting the demand of our customers both in the domestic market as well as abroad as there is a very high demand of soft-gelatin presentation of vitamins in the developed world. Our Soft-Gelatin Capsules Division is another step towards quality, economy, commitment and compliance of our mission statement, which is to ensure the availability of quality drugs at affordable prices throughout the country and the world.
Branded Generic Facilities

ZAFA Sohrab Goth is our most modern and latest Pharmaceutical Formulation Facility. It is one of the largest facilities, area wise in Pakistan, built on an area of 170,000 square feet. This facility is used for the manufacture of following formulation types:
| • Tablets | • Powders and Liquid Vials | • Syrups | • Capsules |
| • Suspensions | • Injections | • Creams & Ointments |
It has one of the most advanced mixing and granulation equipment ensuring complete hand-free operation. With the (single 8.5 hours shift) capacity of 13 million tablets, 130,000 units of syrup/suspension, 1.5 million capsules, 55,000 units of dry suspension, 50,000 Nebulisers, 250,000 ampoules, 100,000 units Sterile Powder vials, 100,000 ointment BFS filled injection 200,000 units. That is 4.6 Billion units per year from this plant alone.
Automatic machines for filling, capping, labeling are used for the purpose to ensure compliance with cGMP Guidelines. In addition final packaging into cartons is also done by Automatic Cartoning Machines. ZAFA Sohrab Goth, has one of the most modern ointment / cream manufacturing facility with a capacity of around 60,000 packs per day.

ZAFA’s facility at Federal “B” Industrial area is the first of all our facilities. This facility has been in operation since 70’s and has been upgraded at regular intervals. The last up-gradation of this facility was carried out in 2005. The total covered area of this facility is around 45,000 square feet and the entire Production, Packaging, Quality Assurance and Quality Control is centrally air-conditioned. This facility is used for the manufacture of following formulation types:-
| • Tablets | • Dry Syrups | • Eye Drops | • Capsules | • Creams & Ointments | • Nasal Sprays |
| • Suspensions | • Aerosols | • Powder and Liquid | • Syrups | • Injections | • Vials |
This is a very diversified facility and has passed all inspections carried out both by national as well as international agencies.
This facility has one of the most advanced mixing and granulation equipment ensuring hand-free operation. The tableting capacity of this facility is around 5.8 million tablets, 72000 units of syrup/suspension, 1.5 million capsules, 40,000 units of dry suspension, 50,000 units of cream/ointment, 40,000 Nebulisers/Nasal drops, 15,000 Aerosols, 100,000 Liquid injectable vials, 410,000 ampoules, 50,000 units of eye drops & 20,000 units of Powder for reconstitution. 2.43 Billion units per year from this plant.
With continuous addition of new equipments, adherence to GMP is ensured. A latest monoblock line for filling ampoules has been added to further increase the capacity of filled ampoules.
Backward Integration

To cater to our ever increasing demand of Printed Packaging material we decided in 2005 to put up our own Packaging facility. Our Printing and Packaging division comprises of state of the art equipment for the printing of cartons/labels/leaflets and all other material required by the facilities in paper or board printing.
The purpose of this facility was to ensure PRINTED material inventory system of Just in Time and at the same time to control cost. This is our second endeavor of backward integration and has already started helping us with preventing stock outs of packaging material and by controlling cost of our cartons and labels. This plant was necessary in order to control cost and stick with our mission of Quality at Economy.
We plan to further expand this facility in the near future by further incorporating other packaging material manufacturing at this site.

Acquired the manufacturing facility of the German I.V. Giants, B1BRAUN, in Pakistan in the year 2000. This facility is based at the HUB Industrial Trading Estate and has been renamed as ZAFA Infusion Division. This facility is one of the few of its kind operating around the world. It has been built on a modular system by one of the most progressive modular organization of Europe. The plant operates on a total hands-free working environment, utilizing the latest BFS (Blow-Fill-Seal) technology, from Rommelag Switzerland. It could be used for the manufacture of 500m1 and 1000m1 Intravenous Infusion bottles. The hand-free operation ensures compliance with latest cGMP guidelines.
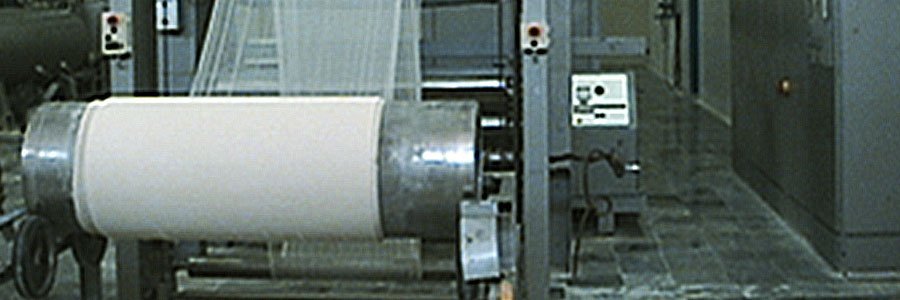
A unit dedicated for the manufacture of Surgical bandages and crepe Bandages. This unit is built as per latest cGMP guidelines and gives ZAFA a complete presence in almost every sector of the health care provider industry. The unit can handle bandages of various sizes and is dedicated to ensure that there is no chance of any cross contaminations. ZAFA Surgitex is equipped with latest machinery from the textile sector and the entire plant is of European origin. Majority of the production of this facility is exported since it is amongst a few facilities at Pakistan to manufacture bandages which are as per Pharmacopoeal specifications. This facility manufactures, crepe and cotton bandages of various sizes.
API Manufacturing Facility

ZAFA celebrated the Moslem festival of EID-ul-FITR of 2005 by purchasing the assets of M/s HIMONT CHEMICAL located at Lahore.
This facility is constructed on an area of 12 acres and the total constructed area is around 45000 square feet. This is a latest facility for the manufacture of Pharmaceutical raw material by way of basic as well as semi-basic manufacturing. This facility is one of the few Pharmaceutical basic raw material manufacturing facility in Pakistan and is fully equipped to handle a variety of products. At the time of acquisition this facility was manufacturing the following products:
| • Paracetamol | • Ciprofloxacin | • Sulphamethoxazole | |
| • Amoxicillin | • Pyrazinamide | • Ampicillin and Other Basic Pharma Raw Materials |
The facility started its commercial operations in April 2007, and initially has started manufacturing Ibuprofen. The product meets all the local & international quality standard and will be major break through in Basic Manufacturing.
ZAFA plans to add more exciting products to the range of already manufactured products at this facility. This facility was providing for the major market share of the products that it manufactured. With our physical presence in Africa and in other countries ZAFA would focus on the global market for the products that we manufacture at our Lahore facility with our strength of manufacturing and marketing Quality Products at economical prices.
ZAFA’s initial target would be to get into the manufacturing and processing of sterile products which would give us further backward integration to existing product lines thus further strengthening us with Quality and Economy.
This facility brings ZAFA closer to our mission statement of ensuring the availability of quality drugs at affordable prices throughout the country and the world.
International Facility

By the Grace of Allah Almighty, ZAFA became the 1st PAKISTAN based Multi-national Pharmaceutical manufacturing concern with its acquisition of major shares of BALSAM Pharmaceutical Company Limited, Sudan. BALSAM Pharmaceuticals, is the largest Pharmaceutical manufacturing concern of SUDAN and is located at NORTH KHARTOUM Industrial Area.
It is the only injectable facility in SUDAN and the only I.V. Infusion facility operating in the KOMESA region. This facility is equipped to manufacture I.V. Infusions, Liquid Injectables in Ampoules and Eye Drops. This facility is in production and ZAFA has sent its technical team to SUDAN to run the operations in addition to our talented local staff at Khartoum. This facility was upgraded by ZAFA after it took over the facility in the year 2004 and has passed inspection by the Sudanese Ministry of Health.
The plant is now being expanded to manufacture Tablets, Capsules, Suspensions, Syrups and Powder and Liquid Injectable Sterile Products thus making it the largest Pharmaceutical facility in the KOMESA region.
The addition of product ranges at BALSAM will help cater to the majority of demand of not only the SUDANESE market but also in the region where ZAFA is recognized as a Quality conscious company. This plant has latest equipment for the manufacturing of IV Infusions, Eye Drops and Liquid Injectables and 90% of the equipments at this facility are of European origin.
ZAFA Pakistan has undergone an inspection carried out by a very high level delegation of the Ministry of Health, Sudan and they have recommended 87 products of ZAFA to be registered in Sudan. ZAFA is signing an agreement with the Government of Sudan to transfer the technical expertise of these products to SUDAN and locally manufacture the maximum number of products at its BALSAM facility.
In addition we are also committed to train at least 2 technical staff of the Ministry of Health Sudan , at ZAFA’s facilities in Pakistan. These facilities have a state of the art Quality Control and Quality Assurance departments which are equipped with latest testing equipments.
Our BALSAM Facility not only makes us the FIRST PAKISTAN BASED MULTINATIONAL PHARMACEUTICAL COMPANY but also is another step towards quality, economy, commitment and compliance of our mission statement which is to ensure the availability of quality drugs at affordable prices throughout the country and the WORLD.
Other Facilities
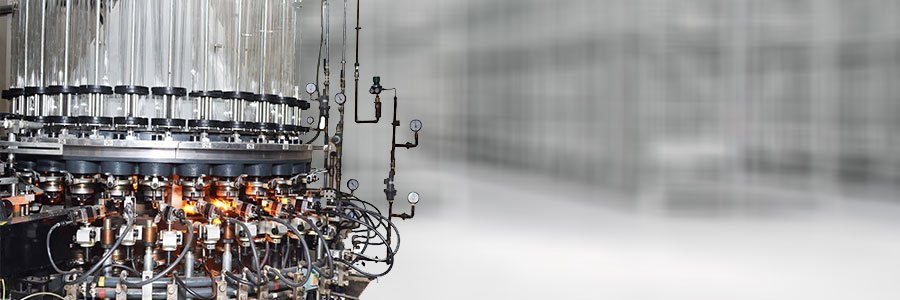
Glastec Ampoules (Pvt.) Ltd. is established to cater to the ever increasing demand of the highest quality glass ampoules by pharmaceutical companies. Our ampoules can be processed providing maximum yield and best quality on any filling line because of their dimensional consistency.
PLANT: We have equipped our plant with the latest, and state of the art ampoule manufacturing lines imported from Europe which have the unique features of dimensional and visual camera and computer controls which no other supplier in Pakistan can offer. We are self reliant in supply of utilities including oxygen. Our in house oxygen generator provides uninterrupted supply of oxygen which is essential for production of glass ampoules. RAW MATERIAL: We are committed to use the highest quality borosilicate USP Type 1 glass tubing from SCHOTT Germany and NIPPON (NEG) Japan to produce ampoules. The glass provides the unmatched quality for the best hydrolytic resistance, alkalinity and consistency. AMPOULES: Ampoules are one of the oldest primary glass packing material. USP Type 1 borosilicate glass is highly stable and the medicine is in contact with glass only which is also 100% tamper proof. These are the features that give glass ampoules a great advantage over its alternatives.
Glastec Ampoule manufactures a wide range of ampoules from 1 ml to 20 ml on its most advanced production lines and highly experienced and devoted staff. Rigorous product inspection and strict process control are ensured through numerous state of the art computer camera control and calibration systems. Glastec Ampoules is the only ampoule manufacturer in Pakistan which is using these systems effectively in their manufacturing process, giving it an edge over any other supplier in Pakistan. Our product range includes flame cut tip (Type B) funnel tip (Type C) and closed tip (Type D) ampoules to DIN and ISO standards. We can also manufacture other custom made ampoules to customer specifications. Ampoules for easy breaking are produced with color break ring CBR or one point cut OPC according to customer’s requirement. Color code rings can be applied for identification purposes. Ampoules can be printed with a wide range of imported glass container enamels / colors which are free of heavy metals.
QUALITY: Glastec Ampoules approach is quality oriented and we aim to keep the quality of our products and services at a level which meets the expectations of our customers. The company is determined to continuously improve the efficiency of its quality system.
The production is monitored by numerous camera control and calibrating systems which inspect every dimensional aspect of products thus reducing our line losses. At the same time our quality control department (QC) also controls all visual, dimensional, physical and chemical parameters of every batch.
US MIL Standard 105E is used to evaluate the lots conformity to laid down standards and release approval is given by QC once all necessary controls have been performed in accordance with internationally accepted guide lines.
The procedures and systems for documentation have been worked out and implemented.
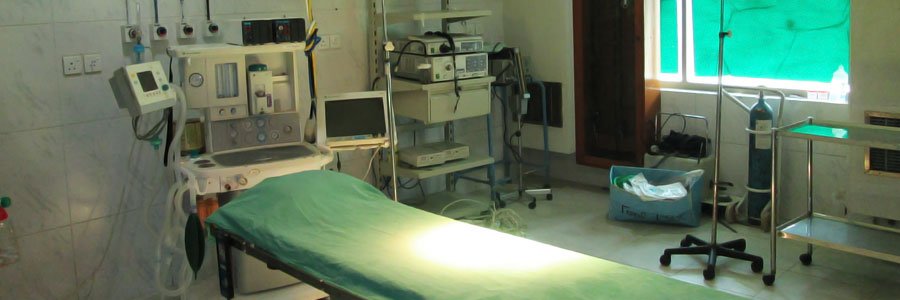
The latest addition to ZAFA GROUP is a 120 bed health facility and named it Boulevard Hospital. Located in the posh district of Defence Housing Authority, Boulevard Hospital provides excellent health care facilities to all the socio economic groups located in the area. With state of the art MRI and CT scan machines the facility boasts operation theaters, CATH labs, and latest Diagnostic equipments. It also houses the best physicians and Surgeons in town.
| • I. C. U. | • C. C. U. | • N. I. C. U. | • CORONARY ANGIOGRAPHY |
| • CORONARY ANGIOPLASTY | • CARDIAC SURGERY | • ORTHOPAEDIC SURGERY | • E. S. W. |
| • LITHOTRIPSY | • P. C. N. L. | • DIALYSIS | • X-RAY |
| • C. T. SCAN | • M.R.I. SCAN | • GYNAE. & OBS. SURGERY | • P. C. N. L. |
| • DENTAL SURGERY | • LAPROSCOPIC SURGERY | • FLUOROSCOPY | • ENDOSCOPY & COLONOSCOPY |
| • BRONCHOSCOPY | • ECHOCARDIOGRAPHY | • E.T.T. | • COLOR DOPPLER |
| • ULTRASOUND | • LABORATORY |
Latest MRI for this facility has been ordered and will be in operation by July, 2017. Furthermore an additional 250 bed hospital is being added on the adjacent plots, thus making it the largest hospital at Defence Housing Authority.


